Help
Study and Reliance Approval Workflow
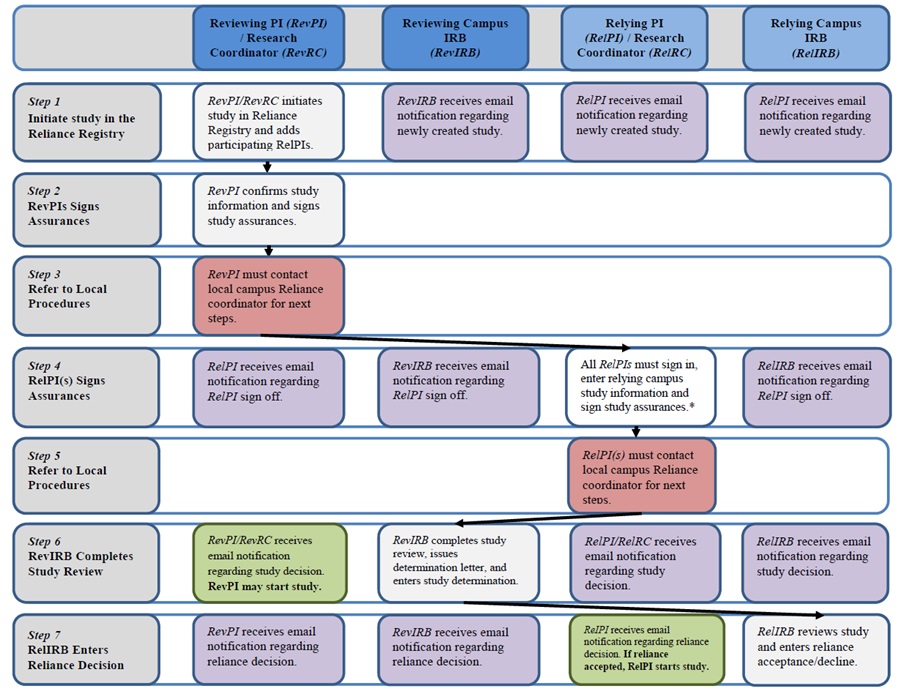
IRB Reliance Registry Overview: Creating a New Study
Glossary
- Reviewing Campus
- Campus conducting the IRB review for a multi-campus human subject study. Usually the prime recipient of the research award, or the UC location where subject contact, recruitment, and/or interactions or interventions entirely or substantially take place.
- Relying Campus
- Campus(es) whose PI participates in the multi-campus study, but does not provide the IRB review and instead chooses to rely on the IRB approval of the Reviewing Campus IRB.
- Reviewing PI
- A PI on the Reviewing Campus. The Reviewing PI submits the study information in the IRB Reliance Registry and to her/his local Reviewing IRB.
- Relying PI
- PI(s) from the campus(es) relying on the Reviewing Campus IRB review.
- Study
- A human subject study that will undergo IRB review. If approved, it will be considered as an active study on the campus where the IRB review took place.
- Reliance
- A reliance is created when an IRB on one campus accepts to rely on the review and approval of a multisite study by the Reviewing Campus IRB.
Registry FAQ
- Q: I am planning a complex collaborative study involving five or more campuses. Where do I start?
- A: In order to get all campus IRBs on the same page, contact your campus IRB Reliance Coordinators before entering your study in the Registry and submitting it for IRB review. In addition to saving you time, the campus IRB Reliance Coordinators will help you set up your complex study in the system in the best format possible. Contact information for the IRB Coordinators is located under the IRB Contacts tab.
- Q: Can my Research Coordinator fill out the Reliance Request form in the Registry for me?
- A: Yes. All you will need to do is review the information your RC entered and assure that everything is correct. As a PI, it is your responsibility to provide correct information about your research.
- Q: Does it mean that the IRB application processes and requirements are now the same on all campuses?
- A: No. The requirements and IRB local application electronic systems were developed independently over time. We recommend that you check with your campus IRB Reliance Coordinator about the reliance requirements on your campus. Contact information for the IRB Coordinators is located under the IRB Contacts tab.
- Q: How do I sign up for the Reliance Registry?
- A: Please sign up via the Registry’s website, on the right side of the homepage. Once you provide your contact information, you will receive an automated email that confirms your user email account and prompts you to complete the registration process.
- Q: What if I have multiple appointments within the University of California system (e.g., UCSF and UC Davis)?
- A: The Registry allows you to choose multiple campuses; however, you will have to designate one campus as your primary location. If you would like to add another UC campus affiliation or change your primary location, you will have to ask your IRB Reliance Coordinator to assist you with editing your user profile.
- Q: How do I enter a study in the Registry?
- A: A multisite human subject study can be entered in the IRB Reliance Registry by a Research Coordinator or by a PI. After logging in to the Registry, you will select a Dashboard tab, answer the three questions, and press “Get Started” button. Once you complete this task, you will assign the respective research coordinators or PIs on your campus (depending on your role), and the Relying PIs .The final step will be to fill out the study details. The arrow markers at the top of the screen will indicate where you currently are in the study set up.
- Q: Why am I unable to enter a study that involves the United States Department of Veterans Affairs (VA)?
- A: At this time VA is not a signatory of the UC IRB MOU.
- Q: Do I have to enter all the study details in one session, or can I return to it and complete it at a later time?
- A: The study details can be edited by a Research Coordinator or Reviewing PI at any stage of completion before the Reviewing PI signs the assurances.
- Q: How do I know when there is a task for me to do in the Registry?
- A: The Registry will send you an email notification (from ORGS-IRBRELIANCE-SA@ucop.edu) to alert you each time a new reliance activity on your study requires your attention. You can also use your To Do List, located under Dashboard tab, to monitor your required actions. The Dashboard also provides you with a clear picture of the pending actions by others on your studies, and it organizes your active and inactive studies. Once you enter your study information, it will move from your “To Do List” to another pending category: Pending PI Signatures, Pending Reviewing IRB Approval, or Pending Relying IRB Acceptance.
- Q: If I am a Reviewing PI, how will I know when my study has been approved?
- A: In addition to informing you through your campus’ IRB’s regular information channels, once your campus IRB approves your study, they will enter the study's expiration date in the Registry, and will upload the Approval Letter. In addition and if applicable, your IRB may also upload the approved protocol, and consent form. You will receive an automated email from the Registry informing you of this step and will be able to review all the uploaded documents in the Registry. As mentioned above, you will also be able to see the study in the Active Studies section of your Dashboard and track the progression of the reliance process for your study at other campuses.
- Q: If I am a Relying PI, how will I know when my study has been approved?
- A: Once the Reviewing campus IRB approves the study, they will enter the study approval's expiration date in the Registry, and will upload the Approval Letter. If applicable, the Reviewing IRB may also upload approved protocol, and consent form, along with any other relevant document for the study. You and your campus IRB will receive an automated email from the Registry, informing you of the Reviewing IRB’s approval and outlining the next steps. Your Relying campus IRB will then be able to review the approval along with all of the uploaded documents, consult with the Reviewing IRB, and choose whether or not they want to rely on the Reviewing IRB’s approval. If they choose to accept to rely, the reliance will be completed. You will receive notification emails at each point in the process but you can also track the progression via the Dashboard: the study will move from the “Pending Reviewing IRB Approval” to “Pending Relying IRB Acceptance” and finally to “Active Study”. Please note that you are not approved to start work on your site until your campus IRB accepts to rely on the Reviewing IRB’s approval. Also note that you are responsible for obtaining all the ancillary committee approvals that are required for your study on your campus.
- Q: If I am a Relying PI, what local processes should I be aware of?
- A: In general, your local IRB office will confirm local ancillary committee approvals, such as Conflict of Interest (COI), Institutional Biosafety Committee (IBC), Radiation Safety, etc. Your local IRB office will also verify that local investigators are eligible PIs. Contact your local IRB office for more information.
- Q: If I am a Reviewing PI/RC, can I use the Reliance Registry to notify my campus’ IRB about any adverse/un-anticipated events?
- A: No, the IRB Reliance Registry should not be used to communicate adverse or unanticipated events to the IRBs. You must communicate any such event directly to your campus IRB using only the regular IRB channels on your campus. Please note that the Relying PIs/RCs are required to promptly inform you of adverse or unanticipated events at their locations which you will have to communicate to your (Reviewing) IRB, using the normal IRB channels as well.
- Q: If I am a Reviewing PI/RC, can I create an amendment or renewal via the IRB Reliance Registry?
- A: Yes, as the Reviewing PI/RC, you may initiate an amendment or renewal in the UC IRB Reliance Registry. Please be aware that both require Reviewing IRB approval. Moreover, please be mindful that no relying site on your study will be able to continue work if you don't obtain IRB renewal on time (i.e., it is your responsibility, as a Reviewing Campus PI, to apply for the renewal on time).
- Q: If I am not a Reviewing PI/RC, can I create an amendment or renewal in the IRB Reliance Registry?
- A: No, only Reviewing PIs/RCs and Reviewing campus IRBs may initiate an amendment or renewal in the UC IRB Reliance Registry. If you are a Relying PI/RC and would like to create an amendment in the UC IRB Reliance Registry, please contact your Reviewing PI.
- Q: I am collaborating with another, non-UC location for my study, do I need to use the Registry for this collaboration?
- A: At this time, the UC IRB Reliance Registry use is based on the Reliance MOU (MOU for IRB Review of Multi-Campus Human Subject Research), signed by the IRB offices on all ten UC campuses and LBNL.
Browsers Supported
IE 8+, Firefox 4+, Chrome 10+, Safari 5.1+ for OSX
